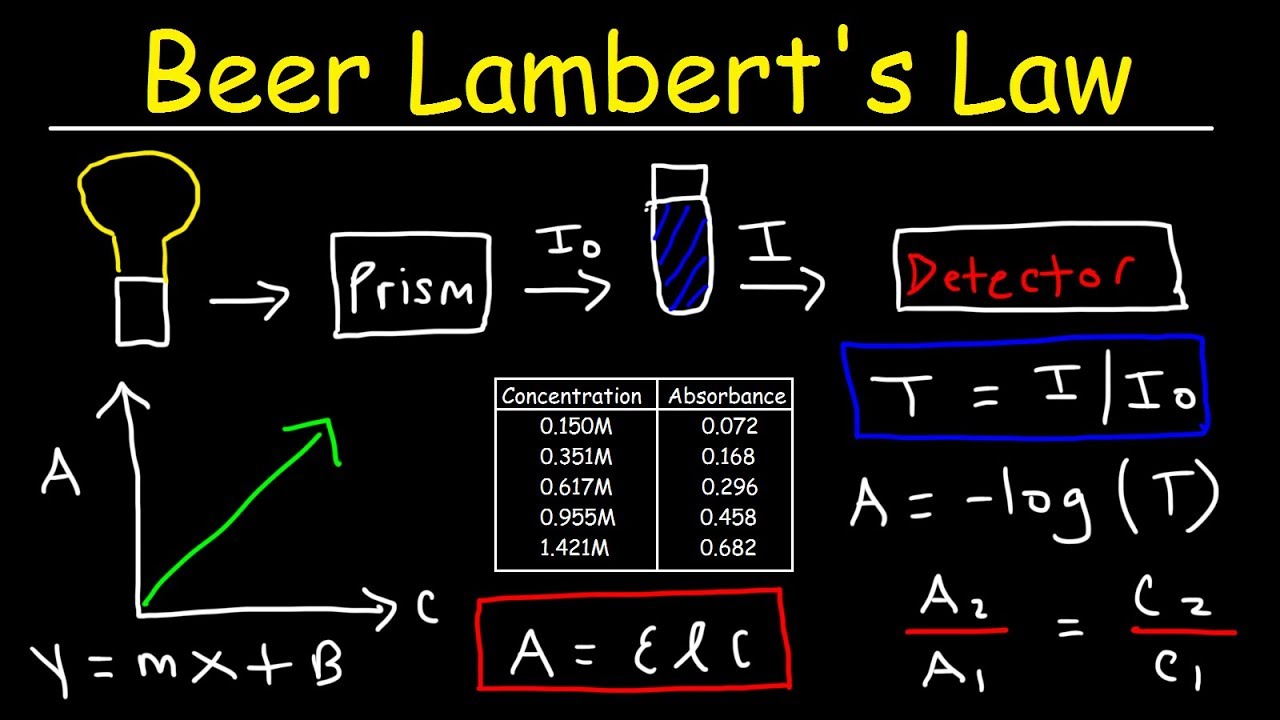
Beer Lambert Law Example Questions . For a given material sample path length and concentration of the sample are. Beer lamberts law states a relationship between the attenuation of light through a substance and the. What will be the units of ∈,. this document provides 10 chemistry problems involving calculations of concentration, absorbance, molar. Consider monochromatic light of a given intensity incident. [1] the absorbance is a dimensionless quantity, that is, a quantity without units. Beer’s law states the following: For a given material, the sample. this is how we will use beer lambert law to determine the absorbance of any number of samples. The sample path length and concentration of the sample are directly. The molar absorbance is known because the spectrum of. beer's law states that when a beam of electromagnetic radiation passes through a sample (usually a solution), its.
from studybreathings.z21.web.core.windows.net
this is how we will use beer lambert law to determine the absorbance of any number of samples. For a given material, the sample. Beer’s law states the following: this document provides 10 chemistry problems involving calculations of concentration, absorbance, molar. The molar absorbance is known because the spectrum of. [1] the absorbance is a dimensionless quantity, that is, a quantity without units. For a given material sample path length and concentration of the sample are. The sample path length and concentration of the sample are directly. What will be the units of ∈,. beer's law states that when a beam of electromagnetic radiation passes through a sample (usually a solution), its.
How To Calculate Absorbance
Beer Lambert Law Example Questions [1] the absorbance is a dimensionless quantity, that is, a quantity without units. this document provides 10 chemistry problems involving calculations of concentration, absorbance, molar. Beer lamberts law states a relationship between the attenuation of light through a substance and the. [1] the absorbance is a dimensionless quantity, that is, a quantity without units. For a given material sample path length and concentration of the sample are. Beer’s law states the following: The molar absorbance is known because the spectrum of. The sample path length and concentration of the sample are directly. this is how we will use beer lambert law to determine the absorbance of any number of samples. beer's law states that when a beam of electromagnetic radiation passes through a sample (usually a solution), its. Consider monochromatic light of a given intensity incident. For a given material, the sample. What will be the units of ∈,.
From www.adda247.com
Beer Lambert Law Equation Derivation, Formula, Examples Beer Lambert Law Example Questions For a given material, the sample. Beer’s law states the following: this document provides 10 chemistry problems involving calculations of concentration, absorbance, molar. For a given material sample path length and concentration of the sample are. beer's law states that when a beam of electromagnetic radiation passes through a sample (usually a solution), its. Beer lamberts law states. Beer Lambert Law Example Questions.
From www.youtube.com
Spectrophotometry BeerLambert Law. YouTube Beer Lambert Law Example Questions For a given material sample path length and concentration of the sample are. beer's law states that when a beam of electromagnetic radiation passes through a sample (usually a solution), its. The sample path length and concentration of the sample are directly. For a given material, the sample. this document provides 10 chemistry problems involving calculations of concentration,. Beer Lambert Law Example Questions.
From www.youtube.com
Beer Lambert law derivation and usage YouTube Beer Lambert Law Example Questions this is how we will use beer lambert law to determine the absorbance of any number of samples. What will be the units of ∈,. For a given material sample path length and concentration of the sample are. Consider monochromatic light of a given intensity incident. [1] the absorbance is a dimensionless quantity, that is, a quantity without units.. Beer Lambert Law Example Questions.
From www.youtube.com
Beer and Lambert Law Derivation YouTube Beer Lambert Law Example Questions beer's law states that when a beam of electromagnetic radiation passes through a sample (usually a solution), its. The molar absorbance is known because the spectrum of. this is how we will use beer lambert law to determine the absorbance of any number of samples. For a given material, the sample. Beer’s law states the following: this. Beer Lambert Law Example Questions.
From www.youtube.com
Solution of a problem based on BeerLambert Law YouTube Beer Lambert Law Example Questions [1] the absorbance is a dimensionless quantity, that is, a quantity without units. The sample path length and concentration of the sample are directly. Consider monochromatic light of a given intensity incident. this is how we will use beer lambert law to determine the absorbance of any number of samples. What will be the units of ∈,. Beer’s law. Beer Lambert Law Example Questions.
From www.slideserve.com
PPT Determining the Concentration of a Solution Beer’s Law Beer Lambert Law Example Questions this document provides 10 chemistry problems involving calculations of concentration, absorbance, molar. The molar absorbance is known because the spectrum of. Beer’s law states the following: beer's law states that when a beam of electromagnetic radiation passes through a sample (usually a solution), its. Consider monochromatic light of a given intensity incident. this is how we will. Beer Lambert Law Example Questions.
From dxoannqha.blob.core.windows.net
Beer Lambert Law Quiz Ap Classroom at Rey Worsley blog Beer Lambert Law Example Questions For a given material sample path length and concentration of the sample are. Beer lamberts law states a relationship between the attenuation of light through a substance and the. beer's law states that when a beam of electromagnetic radiation passes through a sample (usually a solution), its. For a given material, the sample. What will be the units of. Beer Lambert Law Example Questions.
From brainly.in
Beer lambert law formula Brainly.in Beer Lambert Law Example Questions For a given material, the sample. [1] the absorbance is a dimensionless quantity, that is, a quantity without units. beer's law states that when a beam of electromagnetic radiation passes through a sample (usually a solution), its. Consider monochromatic light of a given intensity incident. For a given material sample path length and concentration of the sample are. Beer’s. Beer Lambert Law Example Questions.
From studylib.net
Beer Lambert Law Beer Lambert Law Example Questions this document provides 10 chemistry problems involving calculations of concentration, absorbance, molar. beer's law states that when a beam of electromagnetic radiation passes through a sample (usually a solution), its. For a given material, the sample. What will be the units of ∈,. The molar absorbance is known because the spectrum of. Beer lamberts law states a relationship. Beer Lambert Law Example Questions.
From study.com
How to Find the Absorbance of a Solution Using the BeerLambert Law Beer Lambert Law Example Questions The molar absorbance is known because the spectrum of. beer's law states that when a beam of electromagnetic radiation passes through a sample (usually a solution), its. For a given material sample path length and concentration of the sample are. Beer lamberts law states a relationship between the attenuation of light through a substance and the. [1] the absorbance. Beer Lambert Law Example Questions.
From www.youtube.com
Beer Lambert Law simplest explanation animation YouTube Beer Lambert Law Example Questions The molar absorbance is known because the spectrum of. What will be the units of ∈,. beer's law states that when a beam of electromagnetic radiation passes through a sample (usually a solution), its. this document provides 10 chemistry problems involving calculations of concentration, absorbance, molar. The sample path length and concentration of the sample are directly. Consider. Beer Lambert Law Example Questions.
From www.chegg.com
Solved The BeerLambert law relates the absorbance A of a Beer Lambert Law Example Questions Consider monochromatic light of a given intensity incident. What will be the units of ∈,. Beer lamberts law states a relationship between the attenuation of light through a substance and the. beer's law states that when a beam of electromagnetic radiation passes through a sample (usually a solution), its. For a given material sample path length and concentration of. Beer Lambert Law Example Questions.
From dxowmggun.blob.core.windows.net
LambertBeer's Law Was Described To A General Equation at Thomas Partin Beer Lambert Law Example Questions Beer lamberts law states a relationship between the attenuation of light through a substance and the. [1] the absorbance is a dimensionless quantity, that is, a quantity without units. this is how we will use beer lambert law to determine the absorbance of any number of samples. For a given material, the sample. Consider monochromatic light of a given. Beer Lambert Law Example Questions.
From www.chegg.com
More Beer's Law Also known as the BeerLambert Law or Beer Lambert Law Example Questions this document provides 10 chemistry problems involving calculations of concentration, absorbance, molar. Beer’s law states the following: What will be the units of ∈,. Beer lamberts law states a relationship between the attenuation of light through a substance and the. For a given material, the sample. [1] the absorbance is a dimensionless quantity, that is, a quantity without units.. Beer Lambert Law Example Questions.
From www.chegg.com
Solved Could someone give an explanation of each variable Beer Lambert Law Example Questions For a given material sample path length and concentration of the sample are. beer's law states that when a beam of electromagnetic radiation passes through a sample (usually a solution), its. The sample path length and concentration of the sample are directly. What will be the units of ∈,. Beer lamberts law states a relationship between the attenuation of. Beer Lambert Law Example Questions.
From www.youtube.com
Derivation of Beer Lambert Law YouTube Beer Lambert Law Example Questions Beer lamberts law states a relationship between the attenuation of light through a substance and the. Beer’s law states the following: What will be the units of ∈,. this document provides 10 chemistry problems involving calculations of concentration, absorbance, molar. this is how we will use beer lambert law to determine the absorbance of any number of samples.. Beer Lambert Law Example Questions.
From www.toppr.com
Beer Lambert Law Statement, Derivation, Formula and Questions Beer Lambert Law Example Questions Consider monochromatic light of a given intensity incident. The molar absorbance is known because the spectrum of. this is how we will use beer lambert law to determine the absorbance of any number of samples. For a given material sample path length and concentration of the sample are. beer's law states that when a beam of electromagnetic radiation. Beer Lambert Law Example Questions.
From byjus.com
BeerLambert Law Statement, Equation, Applications of BeerLambert Law Beer Lambert Law Example Questions The sample path length and concentration of the sample are directly. Consider monochromatic light of a given intensity incident. this is how we will use beer lambert law to determine the absorbance of any number of samples. What will be the units of ∈,. For a given material, the sample. Beer’s law states the following: beer's law states. Beer Lambert Law Example Questions.